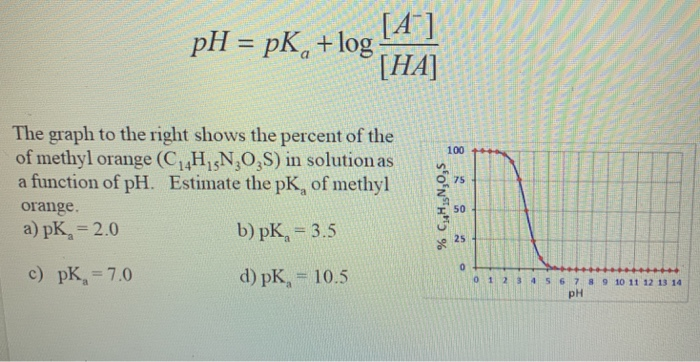
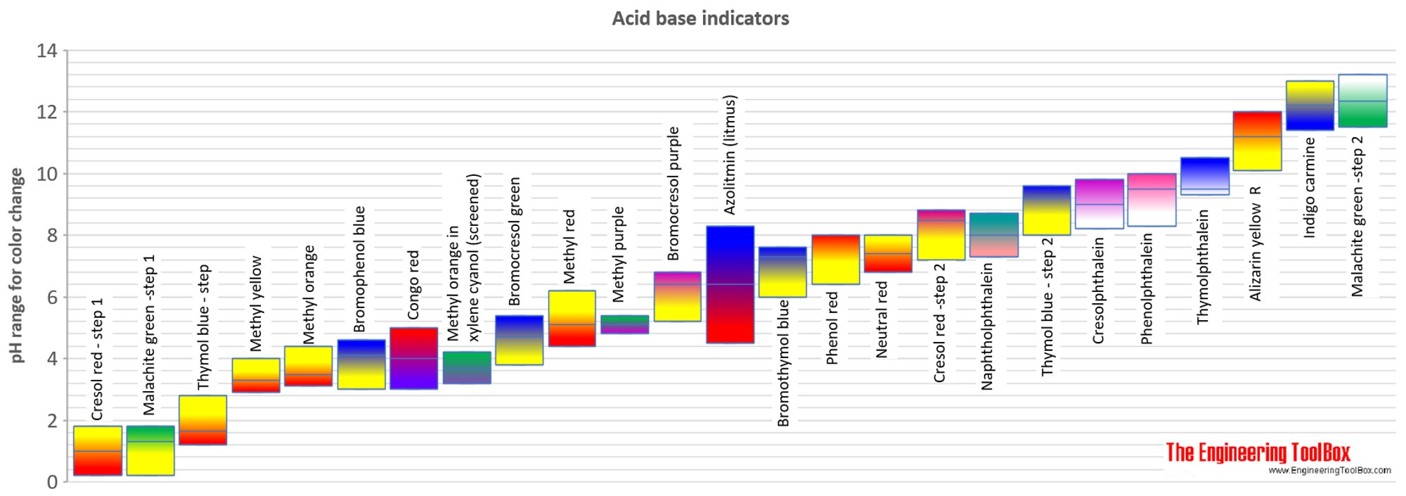
SOLVED:Methyl red has a pKa of 5.0 and is red in its acid form and yellow in its basic form. If several drops of this indicator are placed in a 25.0 -mL

Structures of diazo pH indicators. (A) Main structure of methyl orange... | Download Scientific Diagram
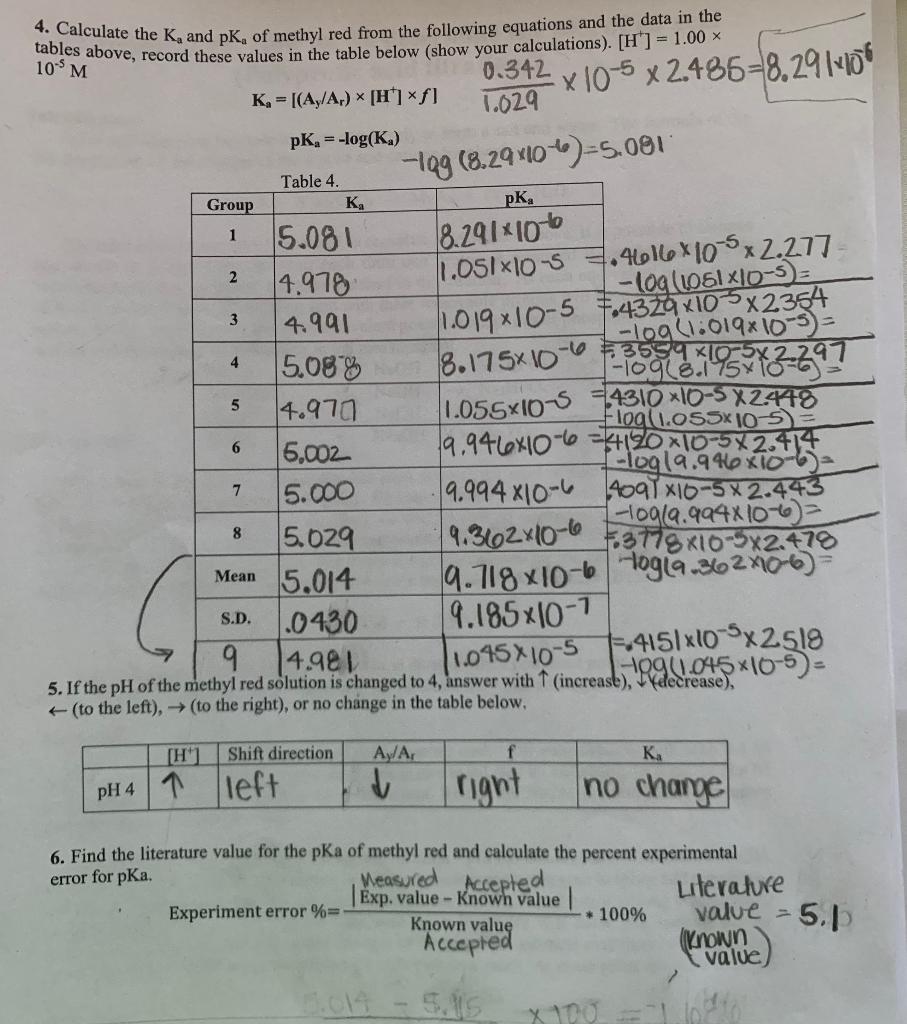
![SOLVED: Calculate the Ka and pKa of methyl red from the following equations and the data in the tables above; record these values in the table below (show your calculations). [H ] = SOLVED: Calculate the Ka and pKa of methyl red from the following equations and the data in the tables above; record these values in the table below (show your calculations). [H ] =](https://cdn.numerade.com/ask_images/a0a70d476fd049fea11245c72f687323.jpg)
SOLVED: Calculate the Ka and pKa of methyl red from the following equations and the data in the tables above; record these values in the table below (show your calculations). [H ] =
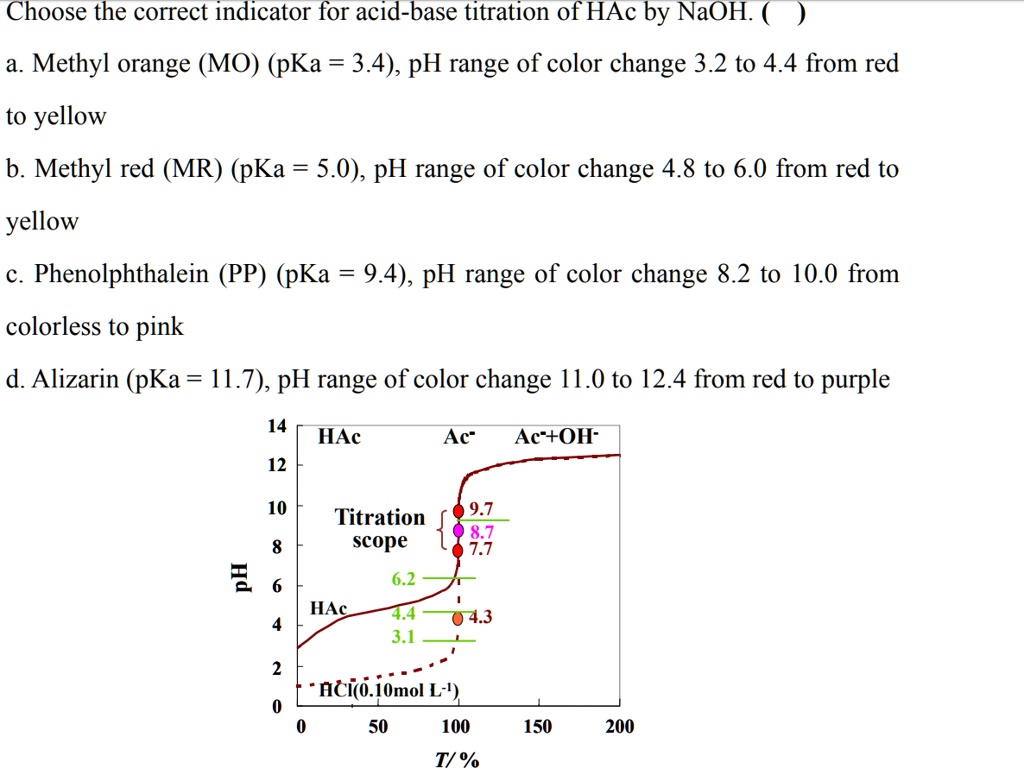
SOLVED: Choose the correct indicator for acid-base titration of HAc by NaOH: Methyl orange (MO) (pKa = 3.4), pH range of color change 3.2 to 4.4 from red to yellow b. Methyl
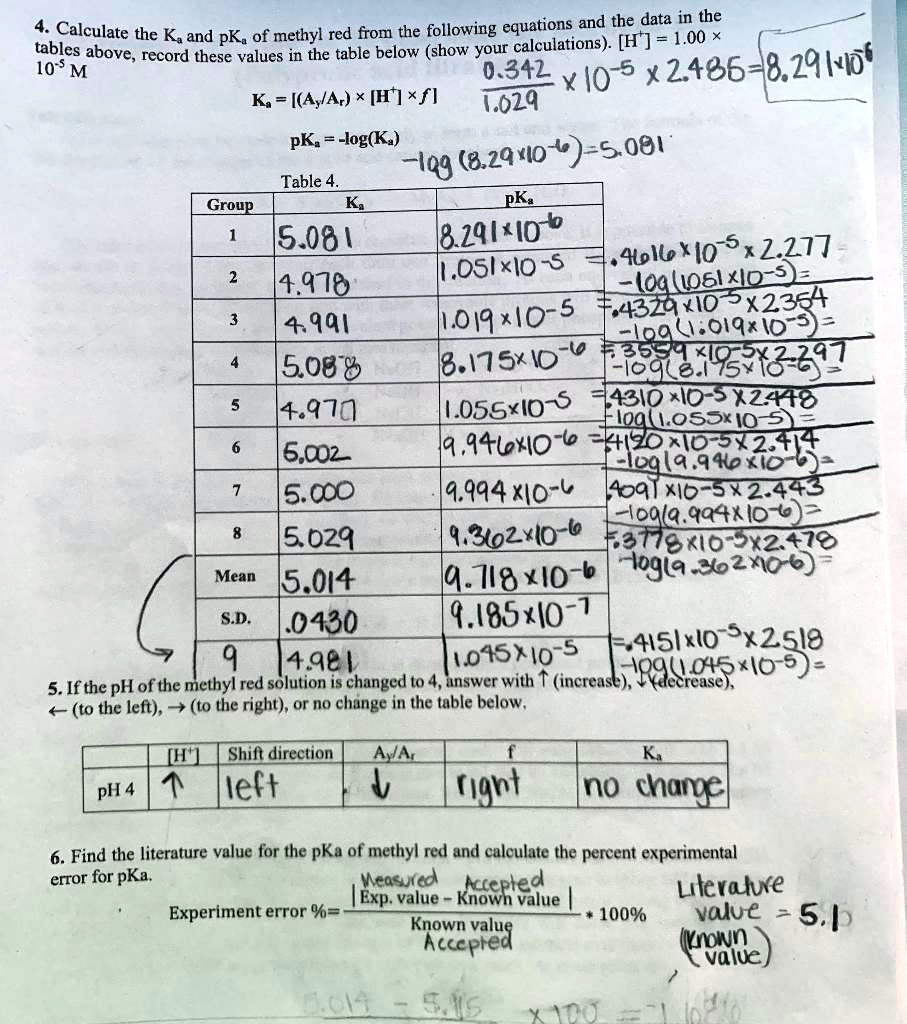
SOLVED: and the data j,8l in the Calculate the K and pK, of methyl red fom the following equationsi tables above, record Tesekvalues €t the cable below (show your calculations) [H7 =

Structures of diazo pH indicators. (A) Main structure of methyl orange... | Download Scientific Diagram

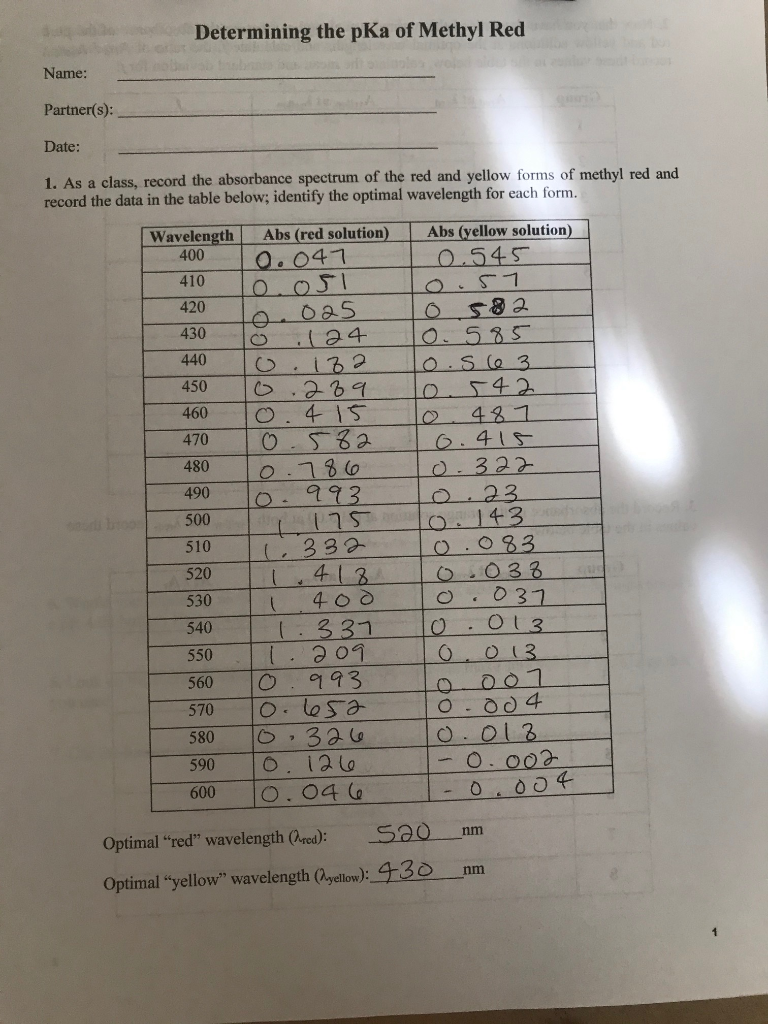
Determination of the Ka of methyl Red Indicator by Colorimetry - Spectroscopy applications: Determination of the Ka of Methyl Red Indicator by | Course Hero

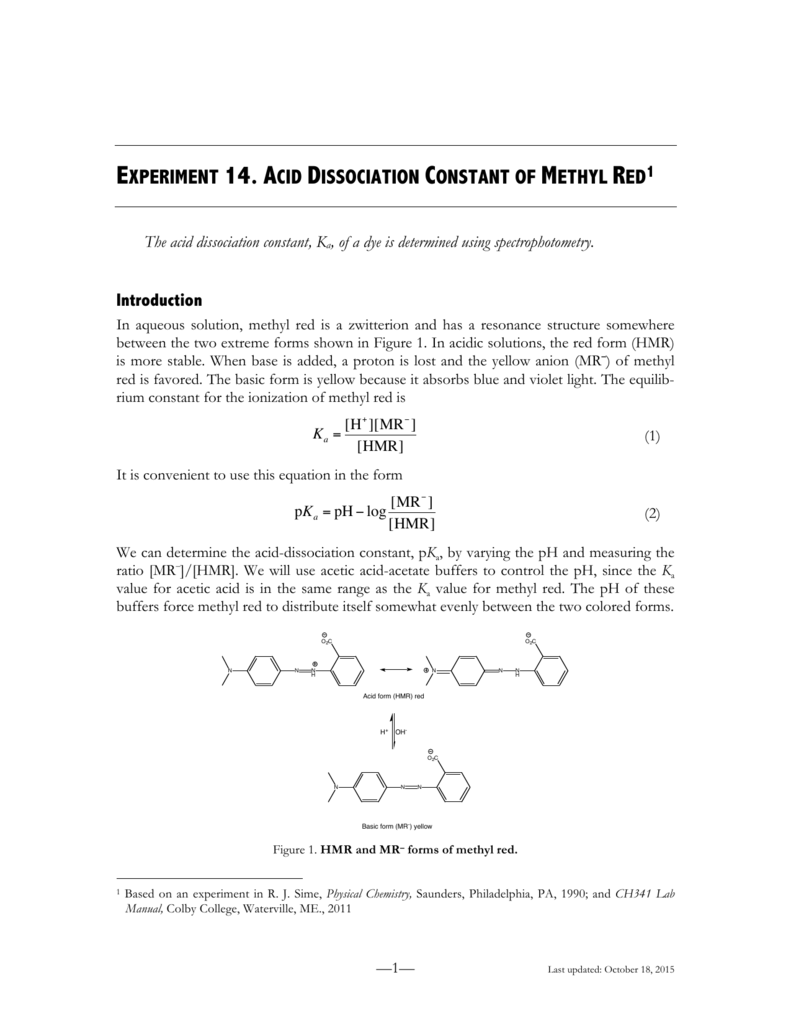
Determination of the Ka of methyl Red Indicator by Colorimetry Discussion - Spectroscopy applications: Determination of the Ka of Methyl Red Indicator | Course Hero