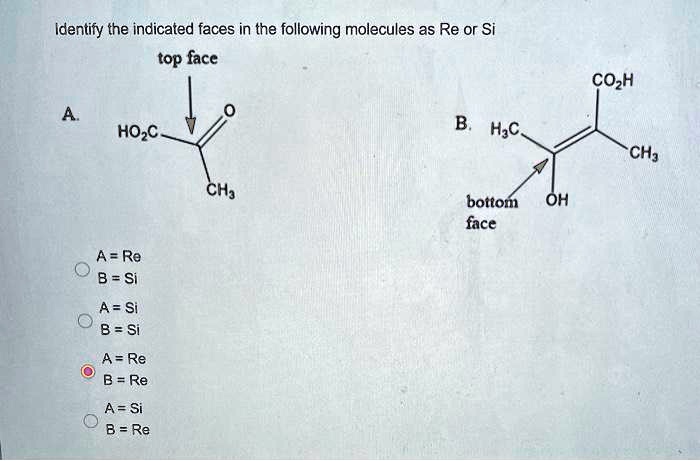
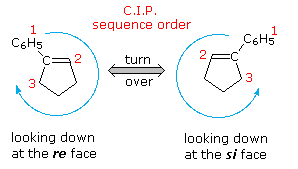
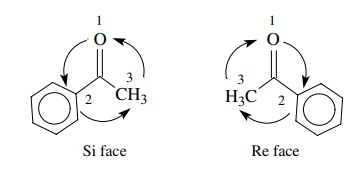
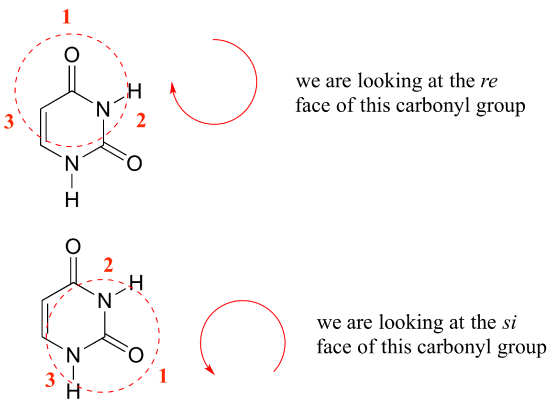
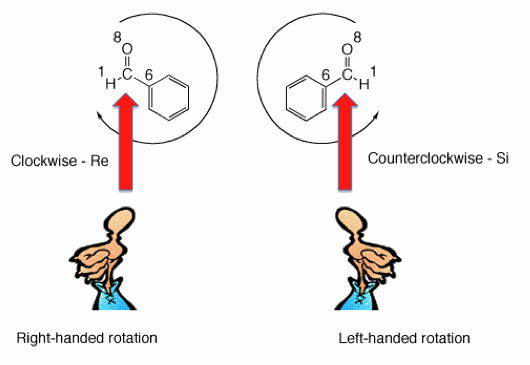
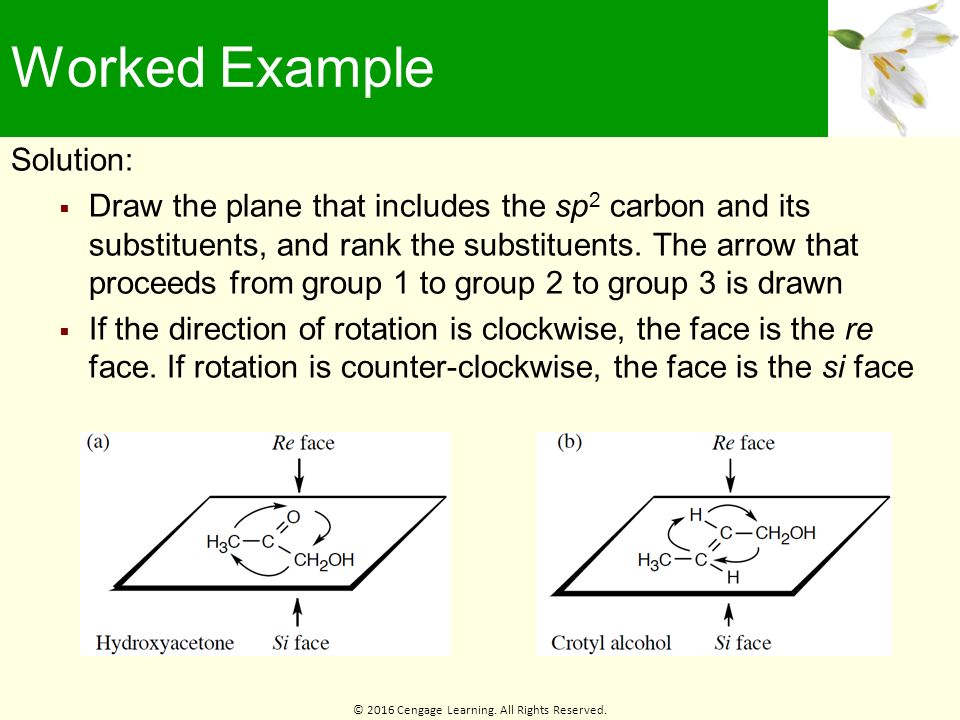
Internal chirality descriptors iR and iS and ire and isi. A proposed notation to extend the usefulness of the R/S system by retaining the sense of stereochemistry in cases of ligand ranking

organic chemistry - Reduction of acetaldehyde from rectus and sinister faces - Chemistry Stack Exchange

Origin of the π‐Facial Stereoselectivity in the Addition of Nucleophilic Reagents to Chiral Aliphatic Ketones as Evidenced by High‐Level Ab Initio Molecular‐Orbital Calculations - Takahashi - 2006 - Chemistry – An Asian

Theoretical study on the mechanism and selectivity of asymmetric cycloaddition reactions of 3-vinylindole catalyzed by chiral N,N'-dioxide-Ni(II) complex - ScienceDirect